Aluminum is the most abundantly available metal on earth. Hence, to use the metal judiciously in different areas of human life multiple industries developed several aluminum alloy components. Moreover, academic programs have also been designed to help candidates meet the industrial workforce requirements. But, one of the most complicated parts of these programs involves the steps to calculate the molar mass of aluminum. If you are stuck in solving a problem on aluminum’s molecular mass and need help to do it correctly, read this blog. Here, we have discussed certain topics such as:
- concept of molar mass,
- chemical and physical properties of aluminum,
- examples of problems in calculating the molar mass of aluminum
- isotope of aluminum
- uses of aluminum
It will give you a comprehensive view of the subject. Read along to learn the details.
What is Molar Mass?
If you are intrigued to learn the steps to calculate the molar mass of aluminum, you must first have a clear concept of what molar mass is.
A mole is a fundamental unit used in chemistry to calculate the amount of substance or anything that has mass and occupies space. You may also call it is as the chemical mass of a substance.
However, molar mass, also known as molecular weight, is the total value of the mass of the atoms in grams present in a molecule, atom, or ions. Just like a standard value like a dozen gives the calculation of 12 items; similarly, a mole measures the quantitative size of the smallest entities. The standard value of the molar mass of a substance is the value of 6.022 x 1023 of a molecule.
Importance of Calculating Molar Mass
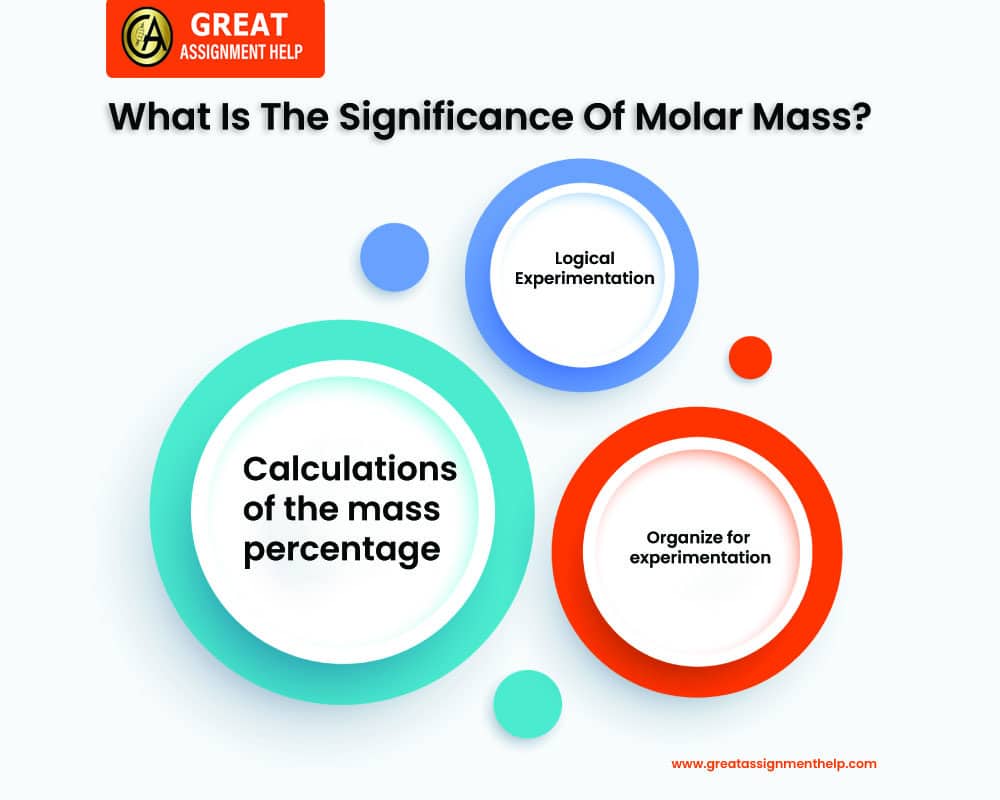
Here are a few reasons why molar mass is important.
Organize for experimentation
When you want to arrange for an experiment, molar mass can be of great help. You can understand how much you should measure on the scale if you are finding values that require specific quantities of a substance (e.g. aluminum) by calculating its molar mass. Imagine an experiment that requires the use of 2 moles of pure aluminum. When you know that aluminum has a molar mass of 26.98 g/mol, you can simply multiply the value by 2 moles to determine the result. You will require 53.96 g of carbon.
Logical Experimentation
The concept of molar mass also helps in examining the investigational results. Suppose you get 2 different substances. Each of them different molecular mass and they take up completely different volumes. Then, how will you understand their molecular size? The simple way is to find the mole that takes up a larger volume. It will have a larger molecular size.
Calculations of the mass percentage
Molar mass is also used to analyze how the percentage of each element in a compound adds to the total mass of that compound. Take a look at the following example: You have a sample of 28.00 g of carbon monoxide. If you know that carbon’s molar mass of carbon is 12.01 g/mol and oxygen’s molar mass is 16.00 g/mol. It indicates that carbon is accountable for 42.89 percent of the total mass (12.01/28.00 times 100).
What is Aluminum?
Before we discuss the steps to calculate the molar mass of aluminum let us know the element aluminum in detail.
Chemical Name: Aluminum (Al).
Weight of Atoms in g/mole: 26.98.
The molecular mass of the aluminum atom: 26.981539 u.
Number of atoms: Aluminum has only one atom. It includes the following:
- A positively charged nucleus consisting of 13 protons,14 neutrons,
- 3 electron shells consisting of 13 electrons
The melting point of aluminum: 660 °C.
The boiling point of aluminum: 2518.82 °C.
Molar volume of aluminum: 10.00 cm³/mol.
Aluminum is a matte white metal, light in weight and malleable in texture. You will find them in the name of Al in group 3 of the periodic table. It has a 13 atomic number and 26.98154 atomic masses.
Today, we know that Danish physicist Hans Christian Orsted has discovered aluminum. However, it is not completely true. Swiss physician Paracelsus first attempted to separate alum from sulfates. He suggested the discovered aluminum was the salt of the earth. Then, in 1959, German doctor and chemist Andrea Libavius could separate alum from the green and blue sulfates and named alum, “alumina”. However, alumina was not famous during that period since the physician could not discover the pure form of aluminum. At that time, the metal was not popular since people did not find it in its pure form. But finally, in 1825, Hans Christian Orsted isolated the metal in the form we know today.
Also read: Excellent Physics Research Topics for Students
Steps to Calculate the Molar Mass of Aluminum
Following are the steps to calculate the Molar Mass of Aluminum.
Step1: Create A Periodic Table
Develop a periodic table. It will help to find out the element’s valence and atomic mass units.
Step 2: Write The Chemical Equation
The second step to calculate the chemical equation of aluminum involves writing the chemical equation. Now, if aluminum has only one element Al, the formula will have one aluminum atom. However, if you have a compound, the equation will be Al2O3. Developing this formula can be quite easy if you know the element valences in the periodic table.
Step3: Note Down The Aluminum’s Valances
The third step mandates you to go back to the periodic table. Then, you must take note of aluminum’s valance. The potency of aluminum in its compounds shows qualities of valence 3. This data will help you in further calculations.
Step4: Find The Number Of Moles In Aluminum
Once you become familiar with the valence, you can identify the count of moles in aluminum. Here, we have considered that there is only one atom. Therefore, the formula will appear as:
M = Ar, Ar (Al) = Al · 1 mol = 26.98 g/mol.
If you have aluminum sulfate as the substance, then the chemical formula will be Al2 (SO4)3. Here, it is clear that aluminum sulfate consists of 3 elements. Hence, you must sum up the masses of the elements. It will give you the mole of the substance.
Step 5: Find The Mass Of One Mole Of Aluminum
Finally, to determine the mass of one mole of aluminum you must use the following formula:
m (Al) = Ðr (Al) / NA = 27 / 6.02 · 1023 = 4.3 · 1023g.
Hence, the mass of one mole of aluminum is 26.98. However, you can round it off to 27 if required.
Sample Problem- Molar Mass of Aluminum Calculation
It is pertinent to know the steps to calculate the molar mass of aluminum when you have to solve a problem on molar mass in an assignment.
Task: Show the dissolution of aluminum oxide.
Solution: On the one hand, you can explain aluminum oxide’s mass as:
MAl2O3 = ρV = ρSh
In this formula:
- V = dissolved aluminum oxide’s volume
- ρ = density of the aluminum oxide
- h = barrier layer’s thickness layer
- S = oxide film’s area
In contrast, the aluminum’s mass in solution is:
Here:
- vAl3 + = the quantity of aluminum;
- cAl3 + = the intensity of aluminum ionic compounds
- mAl = aluminum’s molar mass of aluminum;
- Vp = solution’s volume
- mAl3 + = aluminum’s mass in the solution.
There are 3 oxygen atoms in 2 atoms of aluminum in an aluminum oxide compound. Hence, you can express the mass of aluminum oxide in the form of the mass of metal.
When the following data is available:
ρSh =
Or, h = ≈ 40nm
From this list, you can use phosphoric and hydrochloric acids to dissolve aluminum oxide. It can appear as:
Al2 +6H3PO4 = 2Al(H2PO4)3 + 3H2O
The acid is produced in excess. Therefore, this salt is acidic. However, if this did not take place, the Aluminum phosphate present here stops the dissolution of the acid. Finally, it forms into
Al2O3 + 6HCl = 2AlCl3 + 3H2O
Nonetheless, you cannot dissolve Silicic acid in aluminum oxide because it is itself insoluble in water.
Physical Properties of Aluminum
You can identify aluminum by its following capabilities:
- Conduct electricity
- Conduct heat
- Malleability
- Ductility
- Anticorrosion
- Resistance to frost
For these features, aluminum is used for stamping, forging, drawing, rolling, and welding.
Aluminum is highly susceptible to oxygen. Hence, a thin film of aluminum oxide forms and lasts as long as it is in contact with air. It also stops the metal from consequent oxidation and even develops anticorrosive properties in the metal. Another noteworthy physical property of the metal is its inertness or nonreactive nature to sea and freshwater, organic acids, and concentrated or diluted nitric acid.
Read more: Why Should You Study Physiotherapy Courses?
Chemical Properties of Aluminum
Aluminum is amphoteric. Normally, a thin but strong layer of oxide forms on the metal. It enhances its resistance. However, if you dismantle the oxide layer, aluminum becomes an active and reducing metal. The metal interacts with different other bases and acids under different conditions.
At extremely high heat
When you put it on extremely high heat, it reacts with oxygen.
At high heat
When you heat it at a high temperature, aluminum can react with sulfur, phosphorus, nitrogen, carbon, and iodine.
At normal temperature
At normal temperatures, it mixes with other chemicals like chlorine, bromine, magnesium, and potassium to form chemical compounds.
Exception: Aluminum does not react with hydrogen.
When you purify oxygen from the oxide film it has a strong interaction with water. Reaction with other dilute acids takes place more vigorously. For example, when heated, it interacts with nitric and sulfuric acid concentrations.
Additionally, the amphoteric nature of the metal makes it react with alkalis quite easily. In metallurgy, during the reactions of aluminothermy aluminum reduces itself from the oxides and salts. It produces aluminum (Al), water (H2O) sodium chloride (NaCl), aluminum chloride (AlCl3), hydrogen (H2), and sodium hydroxide (NaOH) as a result of this reaction.
Does Aluminum Have Isotopes?
Aluminum can form an isotope. It is called 27Al. The mass number of the isotope is 27. If you look into its atomic structure you will notice that aluminum has 13 protons and 14 neutrons. Additionally, the isotopes of aluminum are radioactive with mass numbers from 21 and 42. Among these, the isotope 26Al has a long life. The half-life of this isotope is 720 years.
How Do People Get Aluminum?
Aluminum is the most abundant metal on earth but is mostly available in compounds. It is found in 8% of the earth’s surface in the form of rocks and minerals. The foundation of the continents of the earth, or the stone shells is built with aluminosilicates. Apart from that, animal tissues and plant elements also consist of traces of aluminum. However, aluminum is mostly available in the form of bauxite. You will find them easily in tropical and subtropical countries. Plus, you can get aluminum compounds in the form of nepheline. However, you get several other elements while extracting aluminum from this indigenous rock. Potash, soda ash, cement, and fertilizers come out along the way.
Also read: Awesome Philosophy Essay Topics That Will Help You Earn an A+ Grade
Uses Of Aluminum
Aluminum is used for multiple purposes. Here is a glimpse of a few areas.
Metallurgy
People use aluminum as a base for several alloys. Some of the most common ones are:
- AA-8000: developing wire in the National Electrical Code.
- Alclad: aluminum sheet prepared by joining pure aluminum to a high-power core material.
- Al-Li – aluminum and lithium, often used in developing batteries
- Alnico – an alloy of aluminum, nickel, and copper
- Birmabright – an alloy of aluminum, and magnesium
- Duralumin – an alloy of copper and aluminum
Industrial Purpose
People use aluminum alloys in their day-to-day lives and also in construction, architecture, shipbuilding, automotive, space, and aviation technology.
Jewellery and explosive
Aluminum is used in the chemical industry to develop explosives. Apart from that, anodized aluminum is used to make jewelry.
Fabric etching and dyeing
Aluminum is a big player in the textile and tanning industry. Textile mills use aluminum nitrate to etch fabrics before dyeing. Additionally, it is also used in tanning leather.
Manufacturing industry
All electrical product manufacturers use aluminum for developing the filament.
Conclusion
Hopefully from the discussion above you would have gained a clear idea of the steps to calculate the molar mass of aluminum and also have a comprehensive idea of its related topics. But, if you are looking for more detailed information on aluminum molecules, connect with our chemistry assignment for call us immediately.


