Answer: A mixture is a substance consisting of two or more unconnected chemical components. A mixture is a physical combination of two or more substances together in the form of colloids, suspensions, or solutions while maintaining their own identities.
You encounter a variety of mixtures in daily life. As an example, when you breathe in oxygen, nitrogen, and carbon dioxide. As a result, air is a widely distributed mixture.
What is a Mixture?
A mixture is created when two or more components combine with one another without undergoing any chemical changes. The result of the combination of substances is not chemically combined, nor does it lose its identity. Combinations of chemical components, such as elements and compounds are produced when they are mechanically blended or mixed.
In contrast to a compound, the constituents of a mixture do not react chemically to form new material. They just combine and keep their own qualities instead. The lemonade is a mixture since the ingredients are not in fixed amounts. It is still termed lemonade even if it is made using different amounts of lemon juice and sugar.
Examples of Mixtures
- Crude oil is a blend of mostly hydrocarbon-based organic components.
- Seawater is a blend of different types of salt and water.
- Air is a mixture of several gases, including neon, argon, carbon dioxide, oxygen, and nitrogen.
- A mixture of colored dyes constitutes ink.
- Carbon, potassium nitrate, and sulfur are the ingredients of gunpowder.
Properties of Mixtures
Listed below are the properties of mixtures.
- In a mixture, every component maintains its original characteristics.
- It is simple to separate the constituent parts.
- It is varied how much of each component there is.
- Mixtures aren’t chemical; they are physical combinations of several ingredients.
- The boiling and melting points of the mixture are determined by the properties of the constituents.
- A mixture can be created by combining any state of matter whether it be solid, liquid, or gaseous.
- No energy is lost or gained while the mixture is forming.
- There is an infinite range in the ratios of the components that make up the mixture.
What Are the Different Types of Mixtures?
Mixtures can be essentially divided into two groups. These are
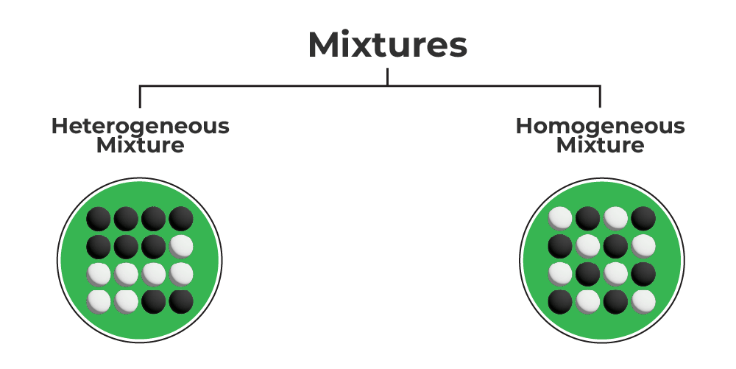
- Homogeneous Mixture
Homo means “the same.” Homogeneous mixtures are those in which the constituents are distributed uniformly throughout the mixture. For example, water and salt are a homogenous mixture as whatever part of the water you sip will taste the same. This indicates that the combination has an even distribution of salt.
In general, a homogeneous mixture can be characterized as follows:
- Each and every compound is a uniform blend.
- No more than one nanometer is present in the particle size.
- Tyndall effect cannot be applied.
- The limits of the elements cannot be separated.
- Neither decantation nor centrifugation can be used to separate the constituent particles.
- Heterogeneous Mixture
A mixture of diverse components that are not homogenous or have localized areas is known as a heterogeneous mixture. Because the components of a heterogeneous mixture do not mix equally, you can identify each one of them separately. Partitioning a heterogeneous mixture into its constituent parts can be done chemically or physically. These combinations are always composed of multiple phases, and their composition varies geographically.
In general, a heterogeneous mixture can be characterized as follows:
- It is evident that the mixture is heterogeneous even without a microscope.
- The distribution of the component particles is not uniform.
- Each component that goes into the combination is identifiable.
- The size of the particles is up to one micrometer.
- Applying the Tyndall effect is possible.
How Can a Mixture Be Prepared?
Mixtures are made using a different procedure. Pharmacists in pharmaceutical factories record prescriptions, preserve records, and adhere to specific proportions. We will look at how to prepare a mixture at home:
Ingredients list:
- One milk glass
- A single tablespoon of butter
- One honey spoonful
- One yolk from an egg
- One-fourth teaspoon of baking soda
One needs to boil a glass of milk before making an egg mixture. Then include honey and a tablespoon of butter. However, you should also incorporate the lightly whisked egg yolk and a little 1/4 teaspoon of baking soda. It is an excellent treatment for tracheitis, laryngitis, and bronchitis in addition to coughs.
How Can Mixtures Be Separated?
Sometimes, the different components of a mixture can be separated into separate units. Below is a list of some techniques for separating mixtures:
- Filtering: Sand combined with water is an example of an insoluble material that can be separated using this technique. Filter paper can be used to pour the mixture through, but the solid will still remain behind because it cannot pass through the paper while the liquid can.
- Evaporation: For the best results, this procedure should be used to separate a mixture of a liquid (often water) and a soluble material that has been dissolved in the liquid. When the solution is brought to a boil, the dissolved material will remain in the solution as the water evaporates.
- Magnetism: This technique functions well when there are a lot of solid metal things. You can distinguish between magnetic and non-magnetic materials by putting a magnet over the mixture of metal objects.
- Decanting: This technique is applicable to combinations of two distinct liquids, such water and oil. Pour the mixture into a container first, then give the liquids time to settle. They should form two distinct layers as they go, which you can then carefully drain out to separate the liquids.
- Sieving: The best use for this technique is sorting different sized solids. Larger items will be left behind in the sieve, while smaller items will flow through the perforations with ease.
In a nutshell, nearly everything in our immediate environment is merely a mixture. A mixture is used in locomotive fuel, much as the food we eat is a variety of substances, and the air we breathe is a mixture of gases.
Leave a Reply