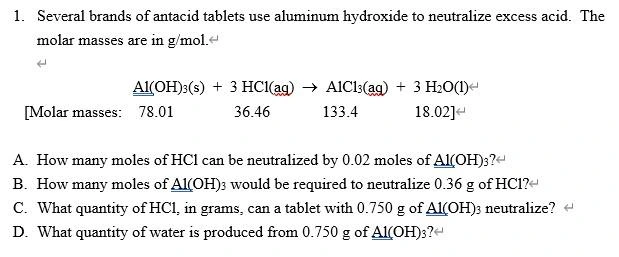
1. Several Brands Of Antacid Tablets Use Aluminum Hydroxide To Neutralize Excess Acid. The Molar Masses Are In G/Mol. Al(OH)3(S) + 3 HCl(Aq) → A1C13(Aq) + 3 H2O(1) [Molar Masses: 78.01 36.46 133.4 18.02] A. How Many Moles Of HC1 Can Be Neutralized By 0.02 Moles Of Al(OH)3? B. How Many Moles Of ALCOH)3 Would Be Required To Neutralize 0.36 G Of HC1?- C. What
Leave a Reply