The drawings below represent aqueous solutions. Solution A is 2.00 L of a 2.00 M aqueous solution of copper(II) nitrate. Solution B is 2.00 L of a 3.00 M aqueous solution of potassium hydroxide.
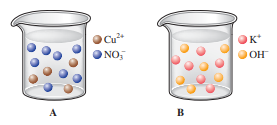
a. Draw a picture of the solution made by mixing solutions A and B together after the precipitation reaction takes place. Make sure this picture shows the correct relative volume compared to solutions A and B, and the correct relative number of ions, along with the correct relative amount of solid formed.
b. Determine the concentrations (in M) of all ions left in solution (from part a) and the mass of solid formed.
Leave a Reply